Risk-Based Quality Management (RBQM): Step-by-Step
- Brien Hawley
- Jun 24, 2025
- 5 min read
Updated: Jun 26, 2025
Here's a primer for RBQM. And here are general steps for implementing RBQM for your study:
Initiate your study-level Quality by Design approach.
Read more about this process here.
A big part of the "Q" in RBQM is determining quality during the design stage, prior to protocol finalization. Once you have completed this process, identified Critical-to-Quality factors and started to identify your Key Risk Indicators, you can move on to your Risk Management Plan...
Assess your risks and implement your Risk Management Plan (RMP).
The process for assessing risks and creating your RMP is outlined in the post here. Essentially, the RMP will include all relevant details of the identified risks, may include CtQ factors and should include Key Risk Indicators. The RMP provides a risk management blueprint that is supported within the additional operational documents below.
Confirm your relevant data points.
This process is probably best managed by someone within your Data Management team. The idea is to take the quality and risk concepts outlined in your protocol and Risk Management Plan and link them to relevant data points that will be captured and analyzed for the study.
Imagine a primary endpoint like "reduced tumor size". The data is collected via a tumor marker test and the results are sent in a data transfer: the field name is TRORRES or "Result of the tumor measurement/assessment as originally received or collected", part of a tumor assessment done by the oncologist. Therefore you have identified the TRORRES data point as "critical" along with the expectations of capturing it at baseline, 2 or more interim visits and end of treatment in order to evaluate response to treatment for a participant.
Once you have identified the data points that link to your CtQ factors, KRIs, QTLs and other important data (e.g. CDISC required or important for analysis), then you can document these within the Data Review Plan...
Implement your Data Review Plan.
A well-built Data Review Plan (DRP) can ensure that checks are in place where they make the most sense, stakeholders are assigned to relevant data review tasks, and there are clear specifications that link back to CtQ factors and critical variables.
At minimum the DRP should include:
Domains or sources of data
Identified key or critical data points that will be checked or reviewed
Nature of checking, e.g. point-of-capture vs. semi-automated vs. manual
Maximize data validation that can be handled through automated checks at the point of capture or on the "back end" (where the team assess the issue first).
Expected, consistent and logical data are often prime targets for more automated checking and cleaning.
Delivery of the check, e.g. through EDC, report review, periodic reconciliation...
Owner, frequency and actions
A "next level" DRP might also include:
Predictive analytics to identify early signals that can impact participant and site success.
Central Statistical Monitoring that leverages concepts found in a Statistical Analysis Plan (Tables, Listings and Figures).
These are then run as data checks during the study to allow proactive management of issues and trends.
Clinical Data Science approaches including AI-driven algorithms to identify trends and outliers, especially unanticipated ones.
Critical windows that trigger actions based on important timeframes or visits.
Implement your Monitoring Plan.
Risk-Based Monitoring (RBM) is a key aspect of RBQM. While central and site monitors could focus on everything -Source Data Verifying (SDV) every data point and investigating every anomaly -it would be much more effective for the monitoring team to focus on critical data and important findings.
Some key questions to help drive the RBM strategy:
Are our CtQ factors covered in the Monitoring Plan? Do they link to relevant data points with expectations around results so that proper SDV and review can be performed?
What KRIs are relevant to monitoring activities and are they included in the SDV or other monitoring tasks?
Are there safety signals (out-of-range values), AEs/SAEs of interest or required safety evaluations that must be documented and present?
Are there data integrity concerns that may be specific to the study and require focused monitoring?
Are there regulatory compliance concerns specific to the study that require focused monitoring?
Within the Monitoring Plan you should review and modify all sections to support a quality-driven risk management approach, not just focusing on SDV or escalated review. Here are a few ideas for quality-driven monitoring:
Observe site activities and request a dry run of the patient screening and enrollment process, as well as a dry run review of key assessments as appropriate.
Prepare for site visits by running key reports and receiving a full analysis from central monitoring. Have a list of required responses ready for the monitoring visit.
While on site, review CtQ factors as they occur, e.g. observe key assessments live and properly document any findings or concerns.
Use pre-defined questions to obtain documented site and participant feedback regarding the study design and experience, especially challenges they are having.
Implement your RBQM systems and tools.
Once you have outlined your plan around monitoring and data review it is time to implement your supporting technologies and tools. There are some RBM and RBQM dedicated platforms worth checking out, and likewise certain reports and configuration options within longstanding clinical systems that can be used to support your RBQM approach.
Here are some ways that you can utilize tools to support RBQM:
Implement targeted SDV or data review within your EDC or other data capture systems that allow for such workflows. This is a way to identify specific data points or critical variables that relate back to your CtQ factors along with other important data (e.g. CDISC, exploratory endpoints, ideal results for analysis...)
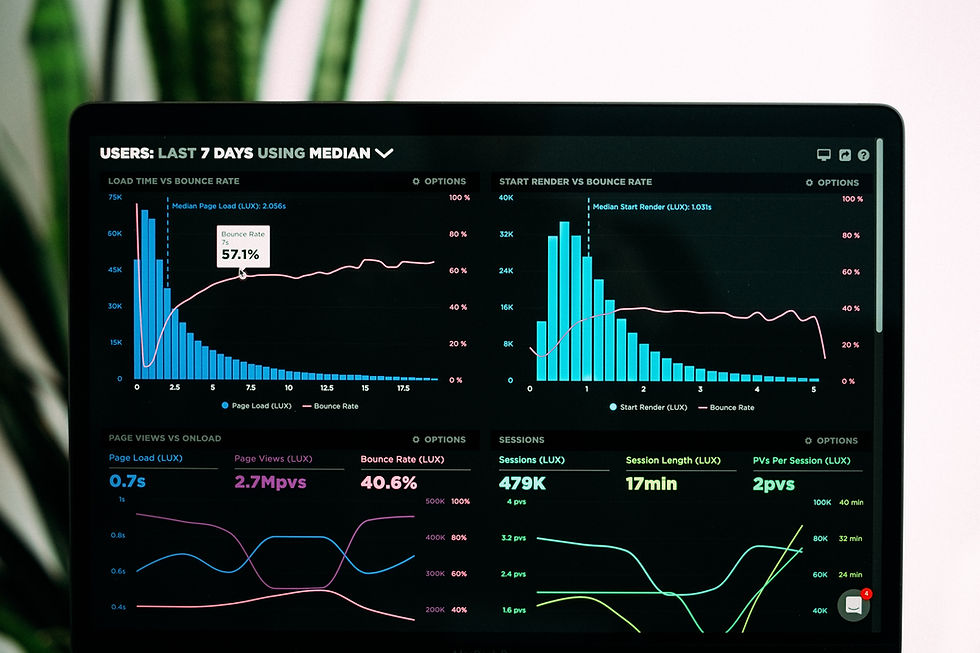
Setup notifications, reports, visualizations and dashboards to output your data anomalies, metrics and KRIs so they can be easily reviewed by assigned stakeholders.
Utilize a RBQM/RBM platform that allows you to configure your agreed monitoring and data review plans into workflows and data outputs.
Do your best with spreadsheets and data listings -while not ideal, these are very low cost alternatives and if implemented the right way can still be effective tools (e.g. utilize simple programming, functions and pivot tables to present relevant fields and anomalies for review).
Learn and evolve.
Quality is not stagnant, it is driven by learning and improving. An important final step as an organization is to learn and drive process changes for improved RBQM in the future. Here are some ways to enable that next level process:
Risk management meetings: Internal risk review meetings that focus on sharing, learning and adding risks (and relevant details) to your risk database. External risk review meetings should evaluate study related risks and remove, edit or add as needed.
Lessons Learned per study: These periodic meetings help the team come together, review KPIs and trends, and discuss lessons that often come about due to risks. Short and long term plans are put in place to address the risks. These meetings can be scheduled on a time series basis, e.g. quarterly, or based on key milestones hit, e.g. 10% of data, 50% of enrollment...
A few example lessons learned questions:
Did we properly define all our CtQ factor data and critical variables, or did we have to modify and why?
Were our reports and outputs clear and actionable, or how can they be improved?
Did our training of sites and participants set proper expectations around completion of the study procedures, or was there confusion and lack of follow through?
Process evolution: Additionally, a critical aspect of continuous improvement is the incorporation of lessons learned into processes moving forward. This could be through updating an SOP, revising a template, adopting a new technology or even building new process from scratch. Whatever it is, make sure it has a timeline and an owner!
Quality, Risks, Actions.
I hope this outline of a basic RBQM process was helpful. Defining your quality factors, key risk indicators and other metrics is an important process, but it is equally important to implement those quality elements into an actionable RBQM process for your study.


Comments